PROTECT YOURSELF with Orgo-Life® QUANTUM TECHNOLOGY
Orgo-Life the new way to the future Advertising by Adpathway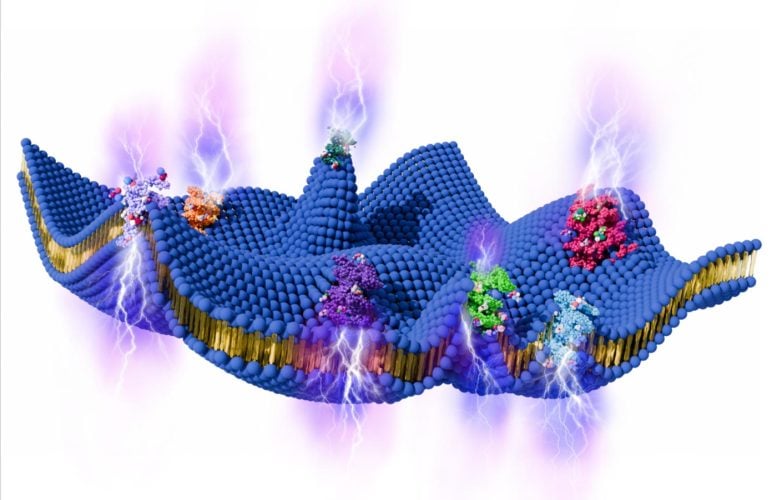 Schematic of an active cell membrane. In a typical active biological process, active proteins (shown in a variety of colors) in a cell membrane (shown in blue) interact with various biological components, such as the ATP molecules (shown in white and red). These interactions of active proteins generate active noise (fluctuation) force within a cell membrane, mechanically affecting the out-of-plane displacement of a cell membrane. Due to the flexoelectric coupling of a cell membrane, changes in out-of-plane displacement induce changes in the transmembrane voltage of a cell membrane, resulting in energy harvesting, active transport of ions, and the generation of electric current across the cell membrane. Credit: Pratik Khandagale, Liping Liu, and Pradeep Sharma
Schematic of an active cell membrane. In a typical active biological process, active proteins (shown in a variety of colors) in a cell membrane (shown in blue) interact with various biological components, such as the ATP molecules (shown in white and red). These interactions of active proteins generate active noise (fluctuation) force within a cell membrane, mechanically affecting the out-of-plane displacement of a cell membrane. Due to the flexoelectric coupling of a cell membrane, changes in out-of-plane displacement induce changes in the transmembrane voltage of a cell membrane, resulting in energy harvesting, active transport of ions, and the generation of electric current across the cell membrane. Credit: Pratik Khandagale, Liping Liu, and Pradeep SharmaLiving cells may generate electricity through the natural motion of their membranes. These fast electrical signals could play a role in how cells communicate and sense their surroundings.
Scientists have proposed a new theoretical explanation for how living cells may generate electrical signals on their own. The idea centers on the cell membrane, the thin, flexible layer that surrounds every cell and separates its interior from the outside environment. Rather than being still, this membrane is constantly in motion due to activity happening inside the cell. The new framework shows that these tiny movements at the molecular level can give rise to real electrical effects.
The work was led by Pradeep Sharma and his colleagues, who developed a mathematical model to connect biological activity with basic physical principles. Their goal was to understand how normal cellular processes could translate into electrical behavior without requiring specialized structures like nerves or electrodes.
Molecular Motion Drives Membrane Fluctuations
Inside living cells, countless processes are always underway. Proteins shift shape as they perform their functions, and chemical reactions release energy that keeps the cell alive. One key process is ATP hydrolysis, which is how cells break down adenosine triphosphate to power biological work. These activities exert forces on the cell membrane, causing it to bend, ripple, and fluctuate.
According to the model, these constant shape changes are not just mechanical. When the membrane bends, it can generate an electrical response through a physical effect known as flexoelectricity. This effect occurs when deformation in a material creates an electrical charge, linking motion directly to voltage.
Voltage Levels Comparable to Neuron Signals
The researchers found that the electrical differences produced across the membrane, known as transmembrane voltages, can be surprisingly strong. In some cases, the voltage may reach up to 90 millivolts. This is similar in size to the voltage changes that occur when neurons send signals in the brain.
The timing of these changes is also striking. The voltage fluctuations can happen over milliseconds, which closely matches the speed and shape of typical action potential curves seen in nerve cells. This suggests that the same underlying physics could help explain how electrical signaling works in biological systems.
Moving Ions Against Their Natural Direction
Beyond generating voltage, the framework predicts another important effect. The electrical signals created by membrane motion could actively move ions. Ions are charged particles that play a central role in cell signaling and maintaining balance inside cells. Normally, ions move along electrochemical gradients, flowing from areas of higher concentration to lower concentration.
The model suggests that active membrane fluctuations could push ions in the opposite direction, effectively working against these gradients. The researchers show that this behavior depends on the membrane’s elastic properties, which describe how easily it bends, and its dielectric properties, which describe how it responds to electric fields. Together, these features determine both the direction and polarity of ion transport.
From Individual Cells to Tissues and Materials
Looking ahead, the authors propose extending this framework beyond single cells. By applying the same principles to groups of cells, scientists could explore how coordinated membrane activity leads to collective electrical behavior at the level of tissues.
The researchers argue that this mechanism offers a physical foundation for understanding sensory perception, neuronal firing, and energy harvesting in living cells. It may also help bridge biological science and engineering by inspiring bio-inspired and physically intelligent materials that mimic the electrical behavior of living systems.
Reference: “Flexoelectricity and the fluctuations of (active) living cells: Implications for energy harvesting, ion transport, and neuronal activity” by Pratik Khandagale, Liping Liu and Pradeep Sharma, 16 December 2025, PNAS Nexus.
DOI: 10.1093/pnasnexus/pgaf362
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.

























 English (US) ·
English (US) ·  French (CA) ·
French (CA) ·  French (FR) ·
French (FR) ·