PROTECT YOUR DNA WITH QUANTUM TECHNOLOGY
Orgo-Life the new way to the future Advertising by Adpathway A team of scientists from Spain, China, and the UK has developed a new nanotechnology approach that reverses Alzheimer’s disease symptoms in mice by repairing the brain’s blood-brain barrier instead of directly targeting neurons. Credit: Stock
A team of scientists from Spain, China, and the UK has developed a new nanotechnology approach that reverses Alzheimer’s disease symptoms in mice by repairing the brain’s blood-brain barrier instead of directly targeting neurons. Credit: StockThe new treatment approach targets restoring normal blood vessel function instead of focusing on neurons or other brain cells, which has been the common strategy until now.
A team of scientists co-led by the Institute for Bioengineering of Catalonia (IBEC) and West China Hospital Sichuan University (WCHSU), in collaboration with UK researchers, has developed a nanotechnology-based treatment that successfully reverses Alzheimer’s disease in mice. Unlike conventional nanomedicine, which typically uses nanoparticles to deliver drugs, this new strategy relies on nanoparticles that are therapeutic themselves, described as “supramolecular drugs.”
Instead of acting directly on neurons, the treatment focuses on repairing the blood-brain barrier (BBB), the protective structure that regulates what enters and exits the brain. By restoring the normal function of this crucial barrier, the researchers were able to reverse Alzheimer’s-related damage in animal models.
The vital role of brain vasculature
The brain is the body’s most energy-demanding organ, requiring about 20% of total energy in adults and as much as 60% in children. This energy is supplied through an extensive network of blood vessels so dense that each neuron is supported by its own capillary. With nearly one billion capillaries in total, the brain’s vascular system is essential for maintaining proper function and protecting against disease. When this system fails, as often seen in dementia and Alzheimer’s, brain health declines rapidly.
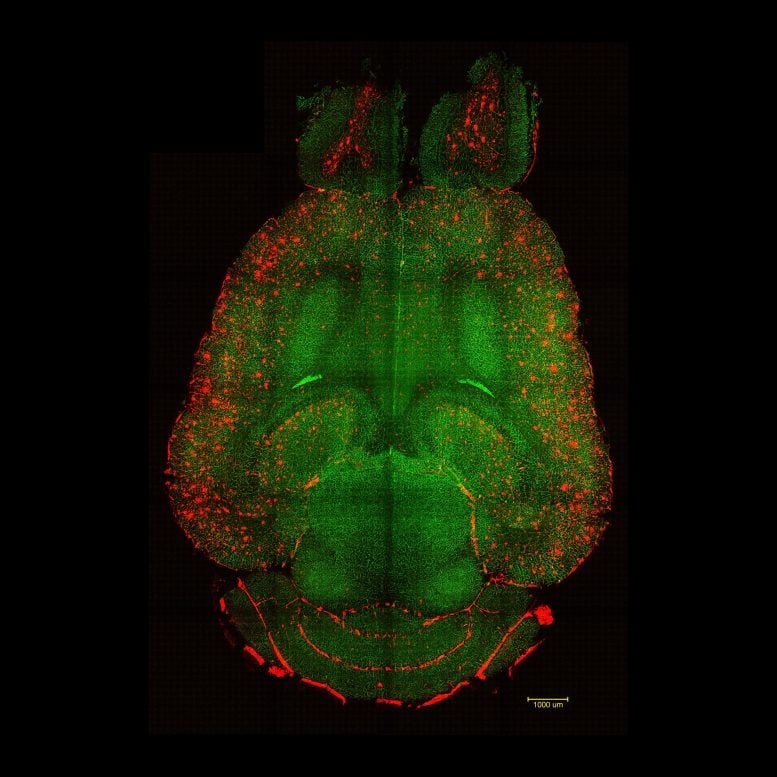 Light sheet fluorescence microscope images of mouse brain 12h after NOT being treated with nanoparticles. The brains were analyzed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)
Light sheet fluorescence microscope images of mouse brain 12h after NOT being treated with nanoparticles. The brains were analyzed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)The blood-brain barrier acts as a gatekeeper between the bloodstream and brain tissue, shielding it from harmful substances such as toxins or pathogens. The research team found that by targeting a specific biological pathway, they could help the brain clear out “waste proteins” that normally accumulate and cause damage. In Alzheimer’s disease, one of the main culprits is amyloid-β (Aβ), a protein that builds up between neurons and disrupts their communication and function.
Reversing Alzheimer’s symptoms in mice
To test the therapy, the scientists used genetically engineered mice designed to produce high levels of Aβ and display cognitive symptoms similar to those seen in Alzheimer’s patients. The mice received just three doses of the supramolecular drugs, after which the researchers closely tracked disease progression.
“Only 1h after the injection we observed a reduction of 50-60% in Aβ amount inside the brain,” explains Junyang Chen, first co-author of the study, researcher at the West China Hospital of Sichuan University and PhD student at University College London (UCL).
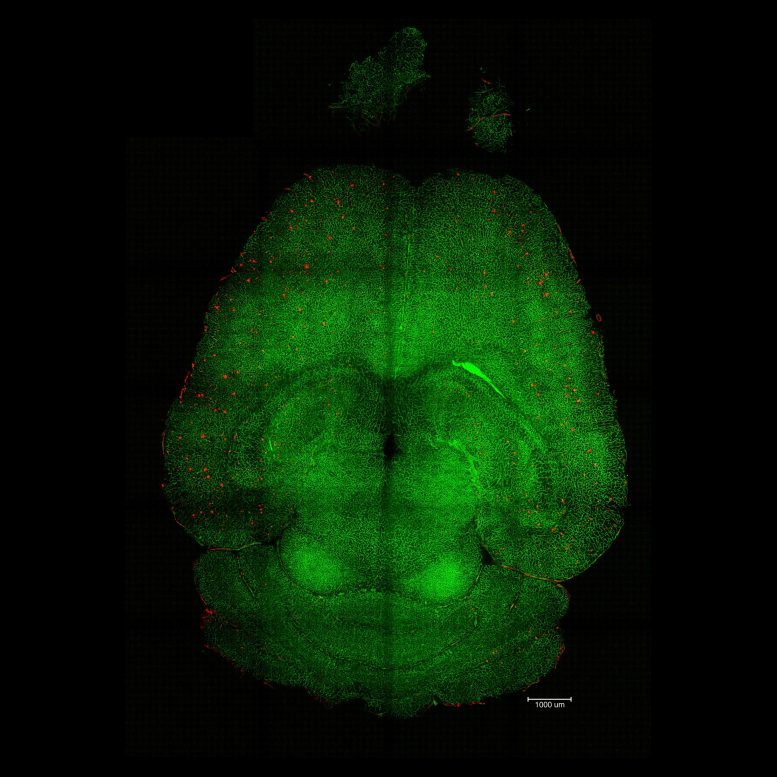 Light sheet fluorescence microscope image of mthe ouse brain 12h after being treated with nanoparticles. The brains were analyzed to see the amount of Aβ plaque accumulation. Red: Aβ plaques. Green: vessels from the blood-brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)
Light sheet fluorescence microscope image of mthe ouse brain 12h after being treated with nanoparticles. The brains were analyzed to see the amount of Aβ plaque accumulation. Red: Aβ plaques. Green: vessels from the blood-brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)The most striking data were the therapeutic effects. Researchers conducted various experiments to analyze the behavior of the animals and measure their memory decline over several months, covering all stages of the disease. In one of the experiments, they treated a 12-month-old mouse (equivalent to a 60-year-old human) with the nanoparticles and analyzed its behavior after 6 months. The result was impressive: the animal, aged 18 months (comparable to a 90-year-old human), had recovered the behavior of a healthy mouse.
“The long-term effect comes from restoring the brain’s vasculature. We think it works like a cascade: when toxic species such as amyloid-beta (Aβ) accumulate, disease progresses. But once the vasculature is able to function again, it starts clearing Aβ and other harmful molecules, allowing the whole system to recover its balance. What’s remarkable is that our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels.”
 Giuseppe Battaglia (left) and Lorena Ruiz Pérez (right). Credit: Institute for Bioengineering of Catalonia (IBEC)
Giuseppe Battaglia (left) and Lorena Ruiz Pérez (right). Credit: Institute for Bioengineering of Catalonia (IBEC)Amyloid-β clearance from the brain
In Alzheimer’s disease, one of the key problems is that the brain’s natural clearance system for toxic species like amyloid-β stops working properly. Normally, the protein LRP1 acts as a molecular gatekeeper: it recognizes Aβ, binds to it through ligands, and ferries it across the blood-brain barrier into the bloodstream, where it can be removed.
https://youtu.be/XzWmyTp2ThA
Statements in English by Giuseppe Battaglia, ICREA Research Professor at the Institute for Bioengineering of Catalonia (IBEC), Principal Investigator of the Molecular Bionics Group and leader of the study. Credit: Institute for Bioengineering of Catalonia (IBEC)
But this system is fragile. If LRP1 binds too much Aβ too tightly, the transport clogs and the protein itself gets degraded inside the brain barrier cells, leaving fewer LRP1 “carriers” available. On the other hand, if it binds too little, the signal is too weak to trigger transport. In both cases, the result is the same: Aβ builds up inside the brain.
The supramolecular drugs developed in this work act like a switch that resets the system. By mimicking the ligands of LRP1, they can bind to Aβ, cross the blood–brain barrier, and initiate the process of removing toxic species from the brain. In doing so, they help restore the vasculature’s natural role as a waste-clearing pathway and bring it back to proper function.
Nanoparticles to treat Alzheimer’s
In this study, the researchers introduce nanoparticles that act as supramolecular drugs, therapeutic agents in their own right rather than carriers of medication. Designed with a bottom-up molecular engineering approach, these nanoparticles combine precise size control with a defined number of surface ligands, creating a multivalent platform able to interact with cellular receptors in a highly specific way.
By engaging receptor trafficking at the cell membrane, they open up a unique and novel way to modulate receptor function. This precision not only enables the effective clearance of amyloid-β from the brain but also restores balance to the vascular system that maintains healthy brain function.
 Xiaohe Tian (left) and Giuseppe Battaglia (right). Credit: Institute for Bioengineering of Catalonia (IBEC)
Xiaohe Tian (left) and Giuseppe Battaglia (right). Credit: Institute for Bioengineering of Catalonia (IBEC)This innovative therapeutic paradigm offers a promising pathway for developing effective clinical interventions, addressing vascular contributions to Alzheimer’s disease, and ultimately enhancing patient outcomes.
“Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance, restoring healthy function in the blood–brain barrier and leading to a striking reversal of Alzheimer’s pathology,” concludes Lorena Ruiz Perez, researcher at the Molecular Bionics group from the Institute for Bioengineering of Catalonia (IBEC) and Serra Hunter Assistant Professor at the University of Barcelona (UB).
Reference: “Rapid amyloid-β clearance and cognitive recovery through multivalent modulation of blood–brain barrier transport” by Junyang Chen, Pan Xiang, Aroa Duro-Castano, Huawei Cai, Bin Guo, Xiqin Liu, Yifan Yu, Su Lui, Kui Luo, Bowen Ke, Lorena Ruiz-Pérez, Qiyong Gong, Xiaohe Tian and Giuseppe Battaglia, 7 October 2025, Signal Transduction and Targeted Therapy.
DOI: 10.1038/s41392-025-02426-1
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google, Discover, and News.






















 English (US) ·
English (US) ·  French (CA) ·
French (CA) ·  French (FR) ·
French (FR) ·