Water ice is everywhere in space, from frozen moons to frosty dust grains in interstellar clouds. However, researchers at University College London (UCL) and the University of Cambridge have recently discovered that ice in space isn't like what we thought it was.
On Earth, the relatively warm temperatures in which ice exists gives the water molecules enough energy to form an ordered, crystalline structure akin to the symmetry of snowflakes. However, in space, temperatures plunge much lower, down to –148 or –328 degrees Fahrenheit (–100 or –200 Celsius) and colder, and it wasn't thought that water-ice could crystallize under such conditions. Instead, water ice in space was thought to be purely amorphous; in other words, no crystallization and no ordered structure between molecules.
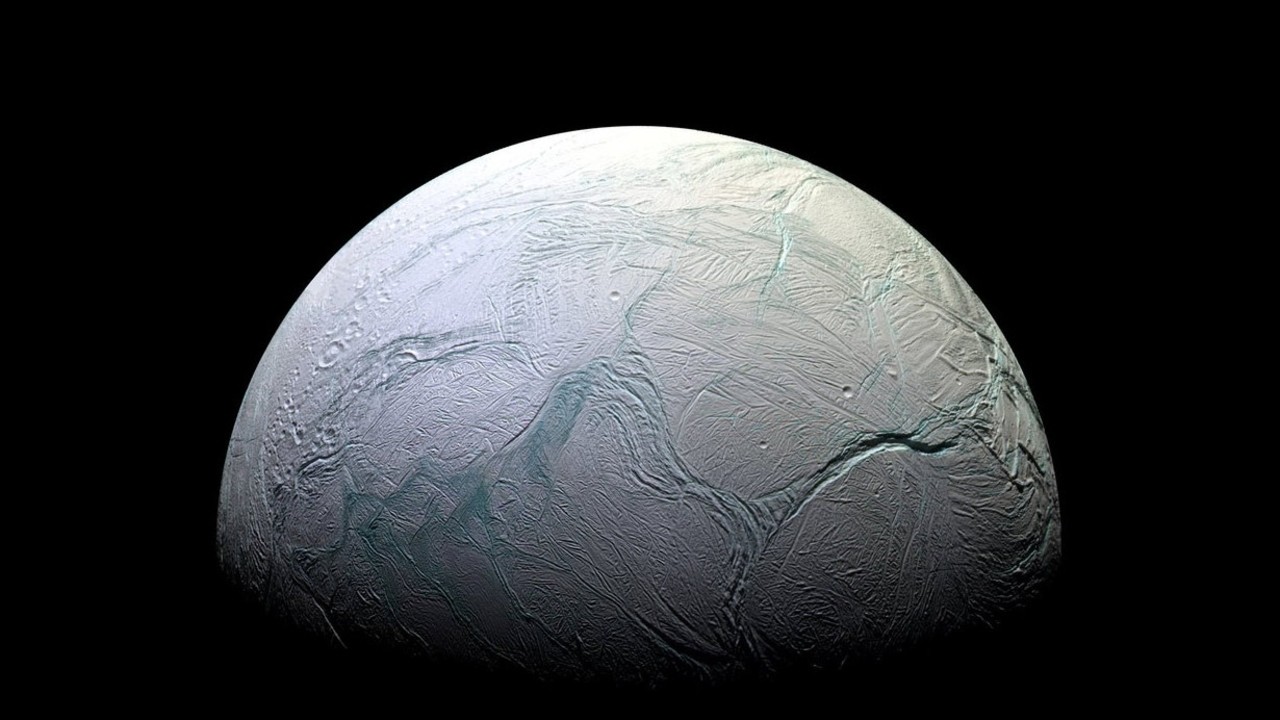
When plumes of water vapor from Saturn's moon Enceladus spew into space, that water vapor freezes and snows back onto the icy moon's surface, but those snowflakes would, according to previous theories, not have the intricate structure of snowflakes on Earth. But now, new research suggests that it could, at least to a degree. Matching computer simulations of how amorphous ice freezes to X-ray diffraction measurements of real amorphous ice suggest that in some cases, up to a quarter of amorphous ice can be made up of crystals.
"We now have a good idea of what the most common form of ice in the universe looks like at an atomic level," said Michael B. Davis of UCL and Cambridge, in a statement. "This is important as ice is involved in many cosmological processes, for instance in how planets form, how galaxies evolve, and how matters moves around the universe."
Low-density amorphous ice was discovered in the 1930s by condensing water vapor onto a metal plate cooled to –166 degrees Fahrenheit (–110 degrees Celsius). High-density amorphous ice was found to be possible in the 1980s when researchers crushed amorphous ice at –328 degrees Fahrenheit (–200 degrees Celsius). And in 2023, Davis' team learned how to create medium-density amorphous ice for the first time. This medium-density amorphous ice has the peculiar property of having the same density as liquid water, so it neither floats nor sinks.
Low-density amorphous ice is most common in the universe, though, and that's what Davis' team used to conduct their experiments.
First, they ran two different simulations. One created virtual ice cubes by cooling water to –120 degrees Celsius (–184 degrees Fahrenheit), but at varied rates so that they froze differently. The other simulation started with large cubes of ice molecules packed together with equal spacing, and then randomly disordered those molecules.
The first simulation produced ice that was not purely amorphous, but was up to 20% crystalline, with the ice crystals measuring just 3 nanometers across and embedding into the disordered gaps within the amorphous ices' structure.
The second simulation managed an even higher percentage of ice crystals, up to 25%.
However, these are still simulations, so how do they stack up to reality? By firing beams of X-rays through supposedly amorphous ice formed through a variety of methods, and watching for how those X-rays were deflected by the molecular structure of the ice, Davis' team found that the amorphous ice's real structure matched that produced by the simulations.
To confirm matters, Davis' team then 're-crystallized' the amorphous ice that they had created, and found that the crystal structure that the ice adopted varied depending upon how the amorphous ice had been formed in the first place. Purely amorphous ice that is totally disordered should retain no memory of its earlier form, unless there is some crystalline structure remaining.
"Ice is potentially a high-performance material in space," said Davis. "It could shield spacecraft from radiation or provide fuel in the form of hydrogen and oxygen. So we need to know about its various forms and properties."
The findings also potentially place constraints on the search for the origins of life, particularly how the building blocks for life came to be on Earth. Some of those organic ingredients for life are thought to have been transported to Earth on icy dust grains, and while these new findings about amorphous ice don't rule that out, they do limit what's possible.
"This is because a partly crystalline structure has less space in which these ingredients could become embedded," said Davis. "The theory could still hold true, though, as there are amorphous regions in the ice where life's building blocks could be trapped and stored."
The research was published on 7 July in the journal Physical Review B.










 English (US) ·
English (US) ·  French (CA) ·
French (CA) ·  French (FR) ·
French (FR) ·